Sunday, 28 February 2016
BIOLOGY NOTES: TRANSPLANTATION:1
BIOLOGY NOTES: TRANSPLANTATION:1: TRANSPLANTATION it is the act of transferring cells, tissues and organs from one site to another. joseph murray in boston performed t...
TRANSPLANTATION:1
TRANSPLANTATION
- it is the act of transferring cells, tissues and organs from one site to another.
- joseph murray in boston performed transplantation for the first time of a kidney between identical twins.
- Today, kidney, pancreas, liver, heart, lungs, bone marrow, and cornea are transplanted between nonidentical individuals too.
- the barrier making transplantation a routine treatment is the immune system.; because it treats the transplanted cells, tissues or organs as foreign .For this, a variety of immunosuppressive treatment is used but due to their effect on the whole immune system; their prolong or intensified use can be deleterious to the recipient.
- So, methods are being developed to induce tolerance to the graft without compromising with the immune responses overall.
Types of Transplants: Degree of immune response to a graft varies with the type of graft.
following are the type: 1) autograft, 2)isograf, 3)allograft, 4)xenograft.
Autograft-it is the transfer of cells or tissues from one place to another within an individual .e.g. transferring healthy skin from one site to another in the burnt patient, and use of healthy blood vessels to replace blocked coronary arteries.
Isograft-in this case, transplantation is carried out between genetically identical individuals. i.e. monozygotic twins in humans or syngeneic mouse.
Allograft-in this case, transplantation is carried out between genetically different members of the same species..e.g. different strains of mouse, two different individuals in humans.
Xenograft-this is the transplantation between different species.. e.g. graft of a baboon heart into a human.
Due to shortage of organs, raising animals for donating organs is under serious consideration.
Graft rejection:
- Autograft and isograft show negligible or no intolerance at all to the graft.
- An allograft being genetically dissimilar generate immune response and is rejected.
- Xenograft exhibit the greatest genetic difference and a vigorous graft rejection.
- The immune response leading to graft rejection shows the attributes of specificity and memory.
- suppose strain A is grafted with skin from strain B. then at first, it might take 12 to 14 days for rejection to occur ; because at first the graft becomes vascularized in few days ;followed by infiltration of graft by immune cells leading to immune response and then decreased vascularization and visible necrosis.
- But, rejection takes only 5-6 days to occur when strain A is again transplanted with skin from strain B; this proves development of memory.
- But these memory cells are specific in their action and this becomes evident when a totally different strain C skin is grafted to strain A; strain C is genetically different from both A and B; and in this case, again strain A mouse takes 12-14 days to reject the graft from strain C because strain A mouse had not developed memory for strain C as it was their first encounter.
Thursday, 25 February 2016
AUTOIMMUNITY 3: COMPONENTS, MECHANISM OF INDUCTION AND TREATMENT
IMMUNE COMPONENTS INVOLVED IN AUTOIMMUNITY
- the autoimmune diseases can involve both the humoural and CMI branch of immune system.
- Out of these, CD4+ T cell has been detected as the primary mediator of autoimmune diseases in a lot of experiments. It has been shown that diseases can be prevented by administering the animals with anti-CD4+ antibodies.
- As we know, T cell recognition of antigen involves a trimolecular complex of the T-cell receptor , an MHC molecule and an antigenic peptide. So, an individual susceptible to autoimmunity must possess MHC molecules and T cell receptors capable of binding self-antigens.
- In most cases of organ specific autoimmune diseases, ratio of T-helper 1 and T-helper 2 cells was seen unbalanced resulting in the development of autoimmunity.
- T-helper 1 has been seen to contribute to the development of disease.;whereas T-helper 2 cells don't only protect against the induction of disease but also against the progression of an already established one.
- Expts. in case of EAE(experimental autoimmune encephalitis) with mouse have proved that when mice were injected with IL-4 at the time of immunization with MBP(meyelin basic protein ) and adjuvant; then the development of EAE was inhibited; whereas injecting IL-12 had opposite effect because IL-4 promotes he development of T-helper (2) cells and IL-12 promotes the development of T-helper (1) cells.
- similarly, association has been detected between a particular type of MHC allele and an autoimmune disease. e.g. HLA-B27 have a 90 times higher likelihood of developing ankylosing spondylitis than individuals with a different HLA-B allele.
- Adding to this, presence of TCRs containing a particular V-alpha and V-beta domains has been linked to a number of disease including EAE and multiple sclerosisand myasthenia gravis.
MECHANISMS FOR INDUCTION OF AUTOIMMUNITY:
There are a variety of mechanisms responsible for the generation of autoimmunity. Autoimmunity doesn't develop from a single event but from anumber of different events. adding to this, susceptibility to autoimmune diseases also differ among different sexes.reasons for induction of autoimmunity:
1)release of sequestered antigens,2)role of molecular mimicry
3)sensitization of autoreactive T cells
4)activation of polyclonal B cells.
1) Release of sequestered antigens. :-
- any tissue antigens that are sequestered from the circulation are not seen by the developing T cells in the thymus and so won't induce tolerance.
- exposure of mature T cells to such an antigen later might result in autoimmunity because they are foreign to the immune system.
- Trauma to tissues following either an accident or a viral or bacterial infection might release sequestered antigens in to the circulation. e.g. sperm arise late in development and are sequestered from the circulation. However, after a vasectomy, some sperm antigens are released into the circulation and can induce auto-antibody formation in some males.
- Similarly, the release of lens proteins after eye damage or of heart muscle antigen after myocardial infarction has been seen to form auto-antibodies.
- It has also been seen that injection of normally sequestered antigens directly into the thymus can reverse the development of tissue-specific autoimmune disease in animals.
2)Role of molecular mimicry:
- A large number of bacteria and viruses possess antigenic determinants that are identical or similar to normal host cell components.
- if immune system has already encountered the similar antigens during maturation; then antibodies are not produced in such cases and this helps microbes to escape the immune response. whereas, if they are similar to sequestered antigens; then auto-antibodies can be formed due to this similarity leading to autoimmunity.e.g- post-rabies encephalitis:- in the past, rabies virus was cultured in rabbit brain cell cultures and vaccine thus prepared included antigens derived from the rabbit brian cells. In a vaccinated person, antibodies against the rabbit brian cell antigen is made which can cross react with recepient's own brian cells leading to encephalitis.
Activation of polyclonal B cells-
many bacteria and viruses induce nonspecific polyclonal B cell activation.This can lead to production of B cells reactive to self antigens.TREATMENT:
- Treating autoimmune diseases is a very difficult deal; because care has to be taken that we are not hampering the whole immune system by targetting just a single disease..
- Besides, current therapies that have been developed so far, target more the symptoms than the disease.
- Immunosuppressive drugs like corticosteroides, azathioprine, and cyclophosphamide are given and they intent to slow down proliferation of lymhocytes. this ,although, reduce the severeity of autoimmune symptoms, but put the patient at a greater risk of infections and cancer.
- Another major limitation is the stage at which disease is identified.earlier the disease is identified, more susceptible it is to the treatment. But, unfortunately, n humans, a disease is diagnosed only after appearence of the symptoms.
- Another problem is the use of animal models; as many treatments have been a succes with animal models but failure in humans.
- so, treatments should be more specific ; the one which can distinguish between a pathologic autoimmune response and a protective immune response. an example is use of cyclosporin or FK506 which block signal transduction mediated by the T cell receptor and so inhibit only antigen activated T cells and spare the nonactivated ones.
practising plasmapheresis:
- it has proven beneficial to patients with autoimmune diseases that involve Ag-Ab complexes. Actually, plasma is removed from patient's blood by continuous flow centrifugation. The blood cells are then resuspended in a suitable medium and returned to the patient. This removes the Ag-Ab complexes present in the patient's blood and give temporary relief. e.g. myasthenia gravis, grave's disease, systemic lupus erythematosus, rheumatoid arthritis.
Targetting inflammation:
- inflammation is a symptoms in many autoimmune diseases and thus, automatically becomes a target.many drugs like humira,remicade, enbrel are products that target TNF-alpha and are used for rheumatoid arthritis, psoriasis etc.
- Antibodies against IL-1R, IL-6R, and IL-15 are being used. Anti-IL-1R is even approved for treatment of rheumatoid arthritis.
use of monoclonal antibody;
- initially, monoclonal antibody directed against CD4 has been used but this deplete all T-helper cells and bring more discomfort than cure. So, not a good idea. ( it was a success in mice to treat multiple sclerosis and arthritis bu a failure in humans.)
- similarly, use of Mab against alpha subunit of IL-2R or CD25 might preferentially block autoreactive T cells.
- as we know, that certain TCR chain components are specifically involved in autoimmune diseases. Targetting them by administering Mab against them might be therapeutic . Same goes with certain allelles of MHC that are specifically associated with certain autoimmune diseases.
Use of oral antigens:
- Different antigens associated with different autoimmune diseases can be administered as vaccines orally preferentially to induce tolerance so that later interaction in any way won't result in autoimmunity.
Wednesday, 24 February 2016
AUTOIMMUNE DISEASES
Autoimmune diseases are of 2 types:
1)organ specific autoimmune disease, 2)systemic autoimmune disease
ORGAN SPECIFIC AUTOIMMUNE DISEASES:
In an organ specific autoimmune disease; the immune response is directed to an antigen unique to a single organ or gland so that the manifestations are largely limited to that organ.cells of a given organ may be damaged in two ways:
1) either by humoural or CMI effector mechanisms,2) Antibodies may overstimulate or block the normal functions of the target organ.
Autoimmune diseases mediated by direct cellular damage:
When lymphocytes or antibodies bind to antigens then cellular lysis or an inflammatory response is generated in the affected organ. Manytimes, the damaged cellular structure is replaced by connective tissues (fibrosis) and the function of the organ declines.e.g.: hashimoto's thyroiditis
Autoimmune anaemias
Goodpasteure's syndrome,
Insulin dependent diabetes mellitus
HASHIMOTO'S THYROIDITIS-
Most frequent in middle aged women; the individual produces autoantibodies and sensitized T-helper 1 cells against thyroid antigens.
As a reslut, DTH response occurs and thyroid gland is infiltrated by lymphocytes, macrophages and plasma cells which form lymphocytic follicles and germinal centers.This leads to hypothyroidism because autoantibodies bind to a number of thyroid proteins and interfere with uptake of iodine. This ,in turn, is manifested by GOITRE.
AUTOIMMUNE ANAEMIAS:
Autoimmune anaemias include :
1)pernicious anaemia 2)autoimmune hemolytic anaemia 3)drug induced hemolytic anaemia1)pernicious anaemia
Intrinsic factor is a membrane bound protein on gastric parietal cells; they help in the absorption of vit.B 12 from small intestine. This vit.B 12 is used in the process of hematopoiesis.
Pernicious anaemia occurs when antibodies are formed to intrinsic factor. As a result, the host becomes incapable of absorbing vit.B12 from small intestine and hematopoiesis is compromised leading to number of functional RBC below normal.Pernicious anaemia is treated sith vit.B 12 injections.
2)Autoimmune hemolytic anaemia
In this case, autoantibodies to antigens present on RBC is made. This leads to complement mediated lysis or antibody mediated opsonization and phagocytosis of RBC ,lowering it's number below normal.3)drug induced hemolytic anaemia -
in this case, drugs such as penicillin or the hypertensive agent like methyldopa interact with RBC and makes it antigenic.To diagnose this disease, RBC is incubated with antibodies against auto-antibodies present in RBC. If agglutination takes place; then presence of autoantibodies is confirmed and patient is diagnosed eith drug induced hemolytic anaemia.
GOODPASTEUR'S SYNDROME:
Basement membrane is found in kidney glomeruli and the alveoli of the lungs and they contain antigens specific to them against which auto-antibodies are produced in this disease .Due to accumulation of auto-antibodies ; complement activation takes place and leads to direct cellular damage and inflammation.
This results in progressive kidney damage and pulmonary haemorrhage. Death may occur after few months.
For diagnosis; biopsies from patients stained with fluorescent labelled anti-IgG and anti-C3b reveal linear deposits if IgG and C3b along the basement membranes.
INSULIN-DEPENDENT DIABETES MELLITUS
it occurs due to autoimmune attack on the beta cells of pancreas due to which insulin production is hampered and blood glucose level increases.At first, beta cells are infiltrated by CTL and macrophages. Activation of macrophages release cytokines. Lytic enzymes of macrophages and cytokines destroys beta cells. Additionally, autoantibodies contribute to cell destruction by facilitating ADCC or antibody mediated complement lysis.
Symptoms : Initially, ketoacidosis and increased urine production occurs; in later stages atherosclerotic vascular lesions may occur which may result in gangrene of extremities due to impeded blood flow, renal failure and blindness.
If untreated, death may occur.
Treatment : insulin injections are administered or transplantation of putified islet cells is equally promising.
AUTOIMMUNE DISEASES DUE TO STIMULATING OR BLOCKING AUTOANTIBODIES:
antibodies may bind harmone receptors instead of ligand and stimulate or block the process concerned . This leads to increase or decrease in the production of mediators and increase in cell growth or gradual atrophy of the affected organ.EXAMPLES OF SUCH DISEASES ARE :
1) greave's disease 2)myasthenia gravis1) GRAVE'S DISEASE
As we know, production of thyroid hormone is regulated by TSH.actually, when TSH binds to receptor on thyroid cell then adenylate cyclase is activated and two thyroid hormones namely thyroxine and tri-iodothyronine is stimulated.
but in case of grave's disease; autoantibodies are produced that bind the receptor for TSH and mimic the normal action of TSH thus producing throid hormones.
but these autoantibodies are not regulated like TSH and consequently, thyroid production is overstimulated and thus overproduced.
for this reason, these antibodies are called LATS antibodies (long acting thyroid stimulating antibodies).
MYASTHENIA GRAVIS
In this case, autoantibodies that bind the acetylcholine receptors present on motor end plates of muscles are produced. they block the normal binding of acetylcholine and the resulting signalling.gradually, the muscles are weakened and complement mediated lysis of cells bearing receptors for these antibodies is caused.
symptoms- drooping eyelids, inability to retract corners of the mouth giving the appearence of snarling.
If not treated, then it can lead to severe impairment of eating and movement problems.
SYSTEMIC AUTOIMMUNE DISEASES:
In this case, response is directed towards a broad range of target antigens and involves a number of organs and tissues.
these diseases cause general defect in immune regulation and result in hyperactive t cells and B cells.
Tissue damage is widespread both due to CMI responses and auto-antibodies and immune complexes.
systemic autoimmune diseases can be exemplified through:
1) systemic lupus erythematosus,
2) multiple sclerosis
3)rheumatoid arthritis
SYSTEMIC LUPUS ERYTHMATOSUS -
More common in women, SLE is characterized by fever, rashes, weakness, arthritis, pleurisy and kidney dysfunction.
Affected individuals produce auto-antibodies to a variety of antigens such as DNA, histone, RBC, platelets, leukocytes and clotting factors. interaction of these antibodies with their respective auto-antibodies leads to variety of symptoms.
e.g. auto-antibodies against RBC and platelets lead to hemolytic anaemia and thrombocytopenia.
immune complexes of auto-antibodies and nuclear antigens are deposited along the wall of small blood vessels leading to type 3 hypersensitivity reaction. these complexes activate the complement system and generate membrane attack complexes which damage blood vessels resulting in vasculitis and glomerulonephritis.
besides, elevated level of complement proteins like C3a and C5a takes place. C5a induces increased expression of CR3 on neutrophiles due to which neutrophiles aggregate and attach to the vascular endothelium. this results in neutropenia and vasculatis leading to severe tissue damage
NOTE: neutropenia is the decrease in the number of neutrophiles and vasculitis is development of various occlusions of the blood vessels.
DIAGNOSIS:antinuclear antibodies are checked for their presence. indirect immunofluoroscent staining with serum from SLE patients produces various characeristics staining patterns.
MULTIPLE SCLEROSIS:
In this case, the cerebrospinal fluid of the patients contain auto-reactive T cells which infiltrate the brain tissue and works to cause inflammation of myelin sheath which insulates nerve fibres. such a breakdown of myelin sheath leads to numerous neurologic dysfunctions.
It is the most common cause of neurologic disability and symptoms may be mild like numbness in limbs to severe like paralysis and loss of vision.
RHEUMATOID ARTHRITIS:
In this case,patients produce a group of auto-antibodies called rheumatoid factors that are reactive with determinants in the Fc region of IgG . the classic rheumatoid factor is and IgM antibody. so, IgM-IgG complexes are formed and deposited in the joints.
These immune complexes activate the complement cascade resulting in a type 3 hypersensitivity reaction which leads to chronic inflammation of the joints which is the major symptom though, respiratory, cardiovascular and hematologic systems are also frequently affected.
Tuesday, 23 February 2016
TOLERANCE AND AUTOIMMUNITY 1
TOLERANCE AND AUTOIMMUNITY
●Sometimes, the immune system fails to distinguish self from nonself and generate response against the self cells too. Such an inappropriate response of the immune system against the self cells is termed AUTOIMMUNITY.
●Autoimmunity leads to a number of chronic and acute autoimmune diseases namely; rheumatoid arthritis, multiple sclerosis, lupus erythematosis and certain types of diabetes.
●But then, there are a number of mechanisms that protect the host from potentially selfreactive lymphocytes . These are given a general term TOLERANCE.
■tolerance is of 2 types:- central and peripheral
■central tolerance- before maturation , T and B cells are exposed to a number of antigens including self antigens presented by medullary thymic epithelial cells and thymic dendritic cells, or bone marrow cells.
●Self-antigens are present due to endogenous expression, importation of antigen from peripheral sites via circulating blood, and in the case of thymic stromal cells, expression of proteins of other non-thymic tissues by the action of the transcription factor AIRE. Those recognising self antigens with a greater than threshold affinity are deleted because if allowed to mature, they will act against self cells leading to autoimmunity.
■How central tolerance acts: It deals with self reactive lymphocytes through clonal deletion ,apoptosis and receptor editing.
●Actually, maturation of lymphocytes to give rise to a functional TCR or Ig occur through a process in which any V-region gene can associate with any D or J segment ; and so generation of a V-region that reacts with a self antigen is possible and so it needs to be checked.
●For this reason, B cells and T cells specific for self antigens are negatively selected in bone marrow and thymus respectively.
self reactive B cells are deleted by the induction of apoptosis and some undergo receptor editing in which the V region is edited and non-autoreactive B cells are released.
Similarly, autoreactive T cells are deleted through apoptosis.
■peripheral tolerance - yet central tolerance fails because:
1)all self antigens are not expressed in the bone marrow and thymus.
2)there is a threshold affinity required between lymphocytes and self antigens to trigger clonal deletion.
●Due to this, weakly self reactive lymphocytes make way to periphery but here peripheral tolerance works to prevent autoimmunity from occurring.
Actually, when in periphery; the B cells get to interact with self antigens; they are inactivated again to prevent them from reaching lymphoid follicles and germinal centers where they become plasma cells able to secrete antibodies .
●If still, they escape: then in the absence of help from T-helper cells which already have been negatively selected in the thymus, they become unresponsive and anergic and never migrate to germinal centers.
●Similarly, T cells in the absence of costimulatory signals cant be activated; Besides, recognition of antigen by TCR presented on MHC is needed.
●Additionally , CTLA-4 acts to inhibity costimulatory signal by binding with B7 and thus inhibiting T cell activation. When gene for CTLA-4 is deleted , the host exhibit autoimmunity .
■ROLE OF T-REGULATORY CELL-weakly self-recognizing T cells are alternatively differentiated into regulatory T cells (Treg cells), which act as sentinels in the periphery to calm down potential instances of T cell autoreactivity.
●T reg cells are a unique type of CD4+ cells that express high levels of the IL-2R alpha-chain (CD25).
Certain of these cells upregulate the transcription factor Foxp3 and then develop in to Treg cells capable of suppressing reaction to self antigens.
Supression is regulated, atleast in part ,through the production of IL-10 and TGF-beta
●Cell death also plays an important role in maintaining tolerance.
Activated T cells express increased levels of Fas and FasL ; In both B and T cells; engagement of fas by fas L leads to AICD ( activation induced cell death).
■NOTE-The deletion threshold is much more stringent for T cells than for B cells since T cells alone can cause direct tissue damage. Furthermore, it is more advantageous for the organism to let its B cells recognize a wider variety of antigen so it can produce antibodies against a greater diversity of pathogens. Since the B cells can only be fully activated after confirmation by more self-restricted T cells that recognize the same antigen, autoreactivity is held in check.
<img class="bm-sh-badge" src="https://www.blogmint.com/blogger/badgeForSefHostedBlog/8254c645e22942838a859e063eeeac14?image=one-pixel.png" alt="badge"/>
Thursday, 18 February 2016
SKIN INFECTIONS :part 2
STREPTOCOCCAL SKIN INFECTIONS :
■INTRODUCTION
●Streptococci are gram (+) bacteria.
●They are highly pathogenic because they have a variety of mechanisms to escape immune defenses of our body . They secrete toxins , enzymes, and virulence factors.
●one of their toxins is HEMOLYSIN , which lyse RBC and almost any type of cell too;
and depending on the hemolysin they produce; streptococci are of 3 types :
1) Alpha-hemolytic
2)Beta-hemolytic
3)Gamma-hemolytic ( it is actually non-hemolytic )
Amon them, beta-hemolytic is often associated with human diseases.
Depending upon the antigenic carbohydrates present on their cell wall ; they have been categorized in to different serological groups from A to T.
The group A streptococci known as GAS are the most important of beta-hemolytic streptococci and are further divided in to 80 immunological types based on the antigenic properties of M proteins present on the cell wall.
■Following properties of GAS makes them pathogenic :
●the M protein prevents activation of complement system and help the microbe to evade phagocytosis.
It also helps the bacteria to adhere to mucous membrane and colonize it.
●Another factor making them virulence is their capsule made up of hyaluronic acid. Actually, hyaluronic acid is poorly immunogenic and few antibodies are made against it.this helps the microbe in better adaptation to the host.
● Besides, GAS also produce substances which helps them in rapid spread of infections through tissue and by liquifying pus.
e.g. STREPTOKINASES-enzymes that dissolves blood clots.
HYALURONIDASE-it dissolves the hyaluronic acid in the connective tissue and helps to
cement together.
DEOXYRIBONUCLEASE- enzymes that degrade DNA.
STREPTOLYSINS- they lyse RBC and are toxic to neutrophiles.
The GROUP A STREPTOCOCCI are synonymous with the species of STREPTOCOCCUS. PYOGENS. some infections caused by S.PYOGENS are as below :
■ERYSIPELAS- it is an infection of the dermal layers of skin. Symptoms are pupules and fever.pupules first occur on the face and then lead to sore throat. It can lead to local tissue destruction and even enter blood stream causing sepsis. It can be treated by beta-lactam antibiotics.
■IMPETIGO- common in childrens and toddlers, symptoms include pustules that become crusted and rupture. This disease is spread by contact and bacteria penetrate the skin thtough cuts and abrasions already present.
■STREPTOCOCCAL TOXIC SHOCK SYNDROME - in case of this TSS bacteremia is more likely to occur than rash. M proteins shade from surfaces of these bacteria forms complex with fibrinogen and binds to neutrophiles. This causes activation of neutrophiles, precipitating the release of damaging enzymes and consequent shock and organ damage.
PSEUDOMONADAL INFECTIONS OF SKIN-:
■pseudomonads are aerobic ,gram (-) bacteria which are capable of growing on unusual organic matter like soap films, and cap liner adhesives.
●the most prominent species is PSEUDOMONAS AERUGINOSA; which is an oppurtunistic pathogen; and causes following types of infections :
1) PSEUDOMONAS DERMATITIS - it is a self-limiting rash of 2 weeks duration and is associated with swimming pools and pool like saunas and hot tubs.
When many people use these facilities; the alkalinity rises and the chlorines become less effective; at the same time, nutritive value of pool to support the growth of microbes increases and P.AERUGINOSA starts flourishing.
Hot water causes hair follicles to open wider and bacteria easily enters inside causing infection.
2)OTITIS EXTERNA ( swimmer 's ear)- it is common among professional swimmers in which the external ear that leads to the eardrum gets infected.
3) IN BURN PATIENTS -P.AERUGINOSA is a serious oppurtunistic pathogen in burn patients and cause blue-green pus on infection; the blue-green colour is due to bacterial pigment PROCYANIN.
■TREATMENTS:
●pseudomonads are resistant to many antibiotics and disinfectants.
But, in recent years, many antibiotics and chemotherapy has been developed. The quinolones and antipseudomonal beta-lactam antibiotics are the drugs of choice.
● silver sulfaduazine is used to treat burn infections by P.AERUGINOSA.
■INTRODUCTION
●Streptococci are gram (+) bacteria.
●They are highly pathogenic because they have a variety of mechanisms to escape immune defenses of our body . They secrete toxins , enzymes, and virulence factors.
●one of their toxins is HEMOLYSIN , which lyse RBC and almost any type of cell too;
and depending on the hemolysin they produce; streptococci are of 3 types :
1) Alpha-hemolytic
2)Beta-hemolytic
3)Gamma-hemolytic ( it is actually non-hemolytic )
Amon them, beta-hemolytic is often associated with human diseases.
Depending upon the antigenic carbohydrates present on their cell wall ; they have been categorized in to different serological groups from A to T.
The group A streptococci known as GAS are the most important of beta-hemolytic streptococci and are further divided in to 80 immunological types based on the antigenic properties of M proteins present on the cell wall.
■Following properties of GAS makes them pathogenic :
●the M protein prevents activation of complement system and help the microbe to evade phagocytosis.
It also helps the bacteria to adhere to mucous membrane and colonize it.
●Another factor making them virulence is their capsule made up of hyaluronic acid. Actually, hyaluronic acid is poorly immunogenic and few antibodies are made against it.this helps the microbe in better adaptation to the host.
● Besides, GAS also produce substances which helps them in rapid spread of infections through tissue and by liquifying pus.
e.g. STREPTOKINASES-enzymes that dissolves blood clots.
HYALURONIDASE-it dissolves the hyaluronic acid in the connective tissue and helps to
cement together.
DEOXYRIBONUCLEASE- enzymes that degrade DNA.
STREPTOLYSINS- they lyse RBC and are toxic to neutrophiles.
The GROUP A STREPTOCOCCI are synonymous with the species of STREPTOCOCCUS. PYOGENS. some infections caused by S.PYOGENS are as below :
■ERYSIPELAS- it is an infection of the dermal layers of skin. Symptoms are pupules and fever.pupules first occur on the face and then lead to sore throat. It can lead to local tissue destruction and even enter blood stream causing sepsis. It can be treated by beta-lactam antibiotics.
■IMPETIGO- common in childrens and toddlers, symptoms include pustules that become crusted and rupture. This disease is spread by contact and bacteria penetrate the skin thtough cuts and abrasions already present.
■STREPTOCOCCAL TOXIC SHOCK SYNDROME - in case of this TSS bacteremia is more likely to occur than rash. M proteins shade from surfaces of these bacteria forms complex with fibrinogen and binds to neutrophiles. This causes activation of neutrophiles, precipitating the release of damaging enzymes and consequent shock and organ damage.
PSEUDOMONADAL INFECTIONS OF SKIN-:
■pseudomonads are aerobic ,gram (-) bacteria which are capable of growing on unusual organic matter like soap films, and cap liner adhesives.
●the most prominent species is PSEUDOMONAS AERUGINOSA; which is an oppurtunistic pathogen; and causes following types of infections :
1) PSEUDOMONAS DERMATITIS - it is a self-limiting rash of 2 weeks duration and is associated with swimming pools and pool like saunas and hot tubs.
When many people use these facilities; the alkalinity rises and the chlorines become less effective; at the same time, nutritive value of pool to support the growth of microbes increases and P.AERUGINOSA starts flourishing.
Hot water causes hair follicles to open wider and bacteria easily enters inside causing infection.
2)OTITIS EXTERNA ( swimmer 's ear)- it is common among professional swimmers in which the external ear that leads to the eardrum gets infected.
3) IN BURN PATIENTS -P.AERUGINOSA is a serious oppurtunistic pathogen in burn patients and cause blue-green pus on infection; the blue-green colour is due to bacterial pigment PROCYANIN.
■TREATMENTS:
●pseudomonads are resistant to many antibiotics and disinfectants.
But, in recent years, many antibiotics and chemotherapy has been developed. The quinolones and antipseudomonal beta-lactam antibiotics are the drugs of choice.
● silver sulfaduazine is used to treat burn infections by P.AERUGINOSA.
SKIN INFECTIONS
SKIN INFECTIONS
SKIN INFECTIONS:
Skin infections are characterized by lesions and rashes but they don’t necessarily be just skin infections and many times are manifestations of some disease of internal organs.
Rashes on the skin are called EXANTHEM and those present on the mucous membrane are called ENANTHEM.
BASED ON THEIR APPEARANCE, following are the types of skin lesion:
VESICLES are small fluid-filled lesions.
When vesicles grow in size of greater than 1 cm diameter then they are called BULLA.
Flat reddened lesions are called MACULES.
When macules are raised then they are called PAPULES.
When papules are filled with pus ,then they are called PUSTULES.
STAPHYLOCOCCI & STREPTOCOCCI are frequent causes of skin infections. Actually, they are well adapted to living in conditions present on skin ,but at the same time are harmful because of enzymes and toxins they produce which makes them pathogenic. Both are gram-positive bacteria and are treated by PENICILLIN antibiotics.
STAPHYLOCOCCAL SKIN INFECTIONS :
For all clinical purposes, staphylococci are divided in to COAGULASE-POSITIVE & COAGULASE -NEGATIVE based on their ability to produce coagulase.
STAPHYLOCOCCUS EPIDERMIDIS-it is a coagulase-negative bacteria and forms 90 % of the normal microbiota of skin. They become pathogenic on getting access to broken skin.
STAPHYLOCOCCUS AUREUS-IT is coagulase positive and forms fibrin clots in blood which protects it from phagocytes and other immune defenses.
Pathogenicity has been seen to be highly correlated to the ability of coagulase production
S.AUREUS also becomes a problem in hospital infections because of extreme use of antibiotics there which makes them resistant and able to cause infection.
Even if the skin is not broken ,then S.AUREUS can enter the body through NASAL PASSAGES & HAIR FOLLICLES.
ĎEPENDING ON IT'S SEVERITY ,INFECTION OF HAIR FOLLICLES IS OF VARIOUS TYPES:
Simple infection of hair follicles is called FOLLICULITIS.(it occur as pimples).
When folliculitis gets more serious then it is called FURUNCLE or BOIL. Furuncle is a type of abscess, which contains pus surrounded by inflamed tissues. It is generally difficult to treat by antibiotics as they cant penetrate it. So,draining pus from the abscess is needed before taking antibiotics for the treatment to be successful.
When furuncle is not treated well then ,neighbouring tissues can be invaded giving rise to CARBUNCLE; which is a hard and deep inflammation of tissues under the skin.
●Infected follicles of an eyelash is called a STY.
IMPETIGO OF THE NEWBORN- it occurs in newborn babies and symptoms are visible as vesicles on the skin. It is treated by HEXACHLOROPHENE containing skin lotions.
■staphylococcal infections often infect underlying tissues and enter the bllod stream where they produce toxins and cause diseases, two of them are discussed below:
●SCALDED SKIN SYNDROME - it is a manifestation of EXFOLIATIVE toxin.It first appears as a lesion sround nose and mouth and rapidly develops and spreads as a bright red area .
Within 48 hours, the skin starts peeling off when touched. It is frequent in kids under 2 years age and vigorous antibiotic therapy is done to protect them.
●TOXIC SHOCK SYNDROME- it is a life-threatening condition and it's symptoms are fever,rashes ,vomiting followed by shock and sometimes organ failure esp. Of kidneys.
It was first discovered in case of staphylococcal growth which occured due to use of a new type of highly adsorbent vaginal tampon.
It is especially high for cases in which tampons remain in place for too long.
Symptoms are caused due to TSST-1 toxin which forms at the growth site and circulates in the blood stream.
Nonmenstrual TSS occurs in cases of women who have recently given birth and nasal surgery because adsorbent packing is used in this case
OUR SKIN
HUMAN SKIN
Skin acts as the first line of defense .
Following properties of it's outer surface makes it an effective barrier:
1.Acidic pH,
2.low moisture
3. Secretion of a peptide antibiotic named defensin.
But larvae of some parasites penetrate the intact skin to get access to our system. Besides, there can be invisible cuts present on our skin that helps them to get inside.
More to this,some parts of our skin like that of armpit and between legs contain high moisture leading to easy growth of microbes and their easy access to our system.
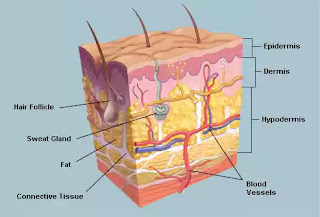
Structure and function of skin-
Skin consists of 2 parts: epidermis and dermis which rest on the subcutaneous fat layer .
Epidermis- it is the thin outer portion composed of several layers of epithelial cells.the outermost portion of skin is dead known as STRATUM CORNEUM.it contains a waterproof protein called KERATIN.
It forms an effective barrier to the entry of microbes.
Dermis-it is inner thick portion of the skin and is composed of connective tissues. Hair follicles,oil glands and sweat glands are present in dermis that can act as passages to the entry of microbes.
NOTE-Perspiration through Sweat glands,though contains nutrients and moisture for microbial growth but they contain salt at the same time which inhibits their growth. It also contain lysozyme.
Sebum secreted by oil glands contains UFA,proteins and salt which, though, nutritive but is inhibitory to the microbial growth at the same time.
NORMAL MICROBIOTA OF THE SKIN-
The surface of our skin is inhospitable to growth of a variety of bacteria and yet satsfying for lot others.reason being that these bacteria can grow in presence of salt and are resistant to drying.
Some aerobic bacteria produce fatty acids from sebum which inhibits the growth of others and help them flourish .
They tend to make clumps so that even vigorous washing can only reduce their number and not eliminate them.they remain in hair follicles and sweat glands and soon reestablish theirselves .
Body odor-areas of the body with high moistures like armpits and the region between legs contain high moisture and microbes present there metabolize the secretions of sweat glands contributing to body odor.
Acne-acne is caused by propionobacterium acne which is a type of diptheroids.they grow on sebum produced by oil glands and make propionic acid which lowers the pH of skin to 3 to 5 and contributes to acne.
Dandruff- dandruff is causef by an yeast named malassezea furfur which grows on sebum and makes skin scaly.shampoos treating dandruff contain KETOCONAZOLE, ZINC PYRITHIONE, SELENIUM SULFIDE which act against yeasts.
Skin acts as the first line of defense .
Following properties of it's outer surface makes it an effective barrier:
1.Acidic pH,
2.low moisture
3. Secretion of a peptide antibiotic named defensin.
But larvae of some parasites penetrate the intact skin to get access to our system. Besides, there can be invisible cuts present on our skin that helps them to get inside.
More to this,some parts of our skin like that of armpit and between legs contain high moisture leading to easy growth of microbes and their easy access to our system.
Structure and function of skin-
Skin consists of 2 parts: epidermis and dermis which rest on the subcutaneous fat layer .
Epidermis- it is the thin outer portion composed of several layers of epithelial cells.the outermost portion of skin is dead known as STRATUM CORNEUM.it contains a waterproof protein called KERATIN.
It forms an effective barrier to the entry of microbes.
Dermis-it is inner thick portion of the skin and is composed of connective tissues. Hair follicles,oil glands and sweat glands are present in dermis that can act as passages to the entry of microbes.
NOTE-Perspiration through Sweat glands,though contains nutrients and moisture for microbial growth but they contain salt at the same time which inhibits their growth. It also contain lysozyme.
Sebum secreted by oil glands contains UFA,proteins and salt which, though, nutritive but is inhibitory to the microbial growth at the same time.
NORMAL MICROBIOTA OF THE SKIN-
The surface of our skin is inhospitable to growth of a variety of bacteria and yet satsfying for lot others.reason being that these bacteria can grow in presence of salt and are resistant to drying.
Some aerobic bacteria produce fatty acids from sebum which inhibits the growth of others and help them flourish .
They tend to make clumps so that even vigorous washing can only reduce their number and not eliminate them.they remain in hair follicles and sweat glands and soon reestablish theirselves .
Body odor-areas of the body with high moistures like armpits and the region between legs contain high moisture and microbes present there metabolize the secretions of sweat glands contributing to body odor.
Acne-acne is caused by propionobacterium acne which is a type of diptheroids.they grow on sebum produced by oil glands and make propionic acid which lowers the pH of skin to 3 to 5 and contributes to acne.
Dandruff- dandruff is causef by an yeast named malassezea furfur which grows on sebum and makes skin scaly.shampoos treating dandruff contain KETOCONAZOLE, ZINC PYRITHIONE, SELENIUM SULFIDE which act against yeasts.
PHAGOCYTOSIS :the process
Understand the introductory phagocytosis here first:
introduction to phagocytosis
Mechanism of phagocytosis
1) PHAGOCYTOSIS consists of 5 steps:
• Chemotaxis
• Adherence
• Ingestion
• Digestion
• Residual body formation
2) Processes in detail:
i. whenever the host is invaded by a microbe .there are a variety of chemical substances that attract phagocytes at the site of infection.(this is CHEMOTAXIS) ........among the chemicals acting as chemotactic substances are microbial products, damaged tissue cells, components of WBC and peptides derived from complement.
ii. after phagocytes are attracted to the target site. They attach their plasma membrane to the surface of microbes or other foreign particles . (This is ADHERENCE )
Adherence can go easy wd some microbes and tough in others...when it's tough then OPSONIZATION occurs in which microbes are coated wd serum proteins like ANTIBODIES and COMPLEMENT PROTEINS leading to their easy adherence and thus phagocytosis.
iii. After attaching to the microbial surface, plasma membrane of the phagocytes extend projections called pseudopods that engulf the microbe. the ends od pseudopodia fuse with each other to form a phagosome which has a very low pH.(this is INGESTION)
iv. Now the phagosome pinches off from the plasma membrane and enters the cytoplasm.; where it fuses with lysosome to form phagolysosome.
Lysosome contains a variety of digestive enzymes and bactericidal substances which become active in the low pH environment of phagosome.(this works to carry DIGESTION)
Lysosomes have lysozymes that hydrolyses the peptidoglycan in bacterial cell walls.
They have lipase ,protease, ribonuclease and deoxyribonuclease that works to digest the macromolecular components of microbes.
They also have enzymes that work to produce toxic oxygen products like hydrogen per oxide ,hydroxide radical, superoxide radical and singlet oxygen by a process called oxidative burst.
v. After digestion is complete, now the phagolysosome contains only the indigestible matter and is known as (RESIDUAL BODY). This residual body moves to the cell surface and releases the waste products outside the cell.
MICROBIAL EVASION OF PHAGOCYTES
Yet some microbes are smart enough to escape phagocytosis and cause further harm. They do this at different levels of phagocytosis. Some escape adherence, others kill phagocytes itself, some survive in low pH and some prevent fusion of phagosome with lysosome.
ESCAPING ADHERENCE:some microbes escape adherence by properties of their outer surface which don't let them adhere to the phagocytes .e.g. M protein in STREPTOCOCCUS PYOGENS and encapsulation in STREPTOCOCCUS PNEUMONIA and HAEMOPHILUS INFLUENZA type b.....but encapsulated microbes can be trapped against a rough surface such as a blood vessel, connective tissue fiber and a blood clot.
ESCAPING KILLING AFTER INGESTION :
proteins like LEUKOCIDINS produced by staphylococci and STREPTOLYSIN produced by streptococci kill phagocytes by releasing the lysosomal enzymes inside the cytoplasm of phagocytes.
A number of pathogens secrete MEMBRANE ATTACK COMPLEXES which first lyse the phagolysosome and then the phagocyte leading to spread of pathogens to neighbouring cells.e.g.TRYPANOSOMA CRUZI-american trypanosomiasis......LISTERIA MONOCYTOGENS -listeriosis
Some microbes have the ability to survive under a low pH .e.g.COXIELLA BURNETTI-Q fever
Some work to escape phagosome before it fuses wd lysosome ...e.g.SHIGELLA-shigellosis....RICKETSSIA-rocky mountain spotted fever.
Some pathogens prevent fusion of phagosome wd lysosome.e.g-HIV-AIDS,...PLASMODIUM-malaria...LEISHMANIA-LEISHMANIASIS.
introduction to phagocytosis
Mechanism of phagocytosis
1) PHAGOCYTOSIS consists of 5 steps:
• Chemotaxis
• Adherence
• Ingestion
• Digestion
• Residual body formation
2) Processes in detail:
i. whenever the host is invaded by a microbe .there are a variety of chemical substances that attract phagocytes at the site of infection.(this is CHEMOTAXIS) ........among the chemicals acting as chemotactic substances are microbial products, damaged tissue cells, components of WBC and peptides derived from complement.
ii. after phagocytes are attracted to the target site. They attach their plasma membrane to the surface of microbes or other foreign particles . (This is ADHERENCE )
Adherence can go easy wd some microbes and tough in others...when it's tough then OPSONIZATION occurs in which microbes are coated wd serum proteins like ANTIBODIES and COMPLEMENT PROTEINS leading to their easy adherence and thus phagocytosis.
iii. After attaching to the microbial surface, plasma membrane of the phagocytes extend projections called pseudopods that engulf the microbe. the ends od pseudopodia fuse with each other to form a phagosome which has a very low pH.(this is INGESTION)
iv. Now the phagosome pinches off from the plasma membrane and enters the cytoplasm.; where it fuses with lysosome to form phagolysosome.
Lysosome contains a variety of digestive enzymes and bactericidal substances which become active in the low pH environment of phagosome.(this works to carry DIGESTION)
Lysosomes have lysozymes that hydrolyses the peptidoglycan in bacterial cell walls.
They have lipase ,protease, ribonuclease and deoxyribonuclease that works to digest the macromolecular components of microbes.
They also have enzymes that work to produce toxic oxygen products like hydrogen per oxide ,hydroxide radical, superoxide radical and singlet oxygen by a process called oxidative burst.
v. After digestion is complete, now the phagolysosome contains only the indigestible matter and is known as (RESIDUAL BODY). This residual body moves to the cell surface and releases the waste products outside the cell.
MICROBIAL EVASION OF PHAGOCYTES
Yet some microbes are smart enough to escape phagocytosis and cause further harm. They do this at different levels of phagocytosis. Some escape adherence, others kill phagocytes itself, some survive in low pH and some prevent fusion of phagosome with lysosome.
ESCAPING ADHERENCE:some microbes escape adherence by properties of their outer surface which don't let them adhere to the phagocytes .e.g. M protein in STREPTOCOCCUS PYOGENS and encapsulation in STREPTOCOCCUS PNEUMONIA and HAEMOPHILUS INFLUENZA type b.....but encapsulated microbes can be trapped against a rough surface such as a blood vessel, connective tissue fiber and a blood clot.
ESCAPING KILLING AFTER INGESTION :
proteins like LEUKOCIDINS produced by staphylococci and STREPTOLYSIN produced by streptococci kill phagocytes by releasing the lysosomal enzymes inside the cytoplasm of phagocytes.
A number of pathogens secrete MEMBRANE ATTACK COMPLEXES which first lyse the phagolysosome and then the phagocyte leading to spread of pathogens to neighbouring cells.e.g.TRYPANOSOMA CRUZI-american trypanosomiasis......LISTERIA MONOCYTOGENS -listeriosis
Some microbes have the ability to survive under a low pH .e.g.COXIELLA BURNETTI-Q fever
Some work to escape phagosome before it fuses wd lysosome ...e.g.SHIGELLA-shigellosis....RICKETSSIA-rocky mountain spotted fever.
Some pathogens prevent fusion of phagosome wd lysosome.e.g-HIV-AIDS,...PLASMODIUM-malaria...LEISHMANIA-LEISHMANIASIS.
Wednesday, 17 February 2016
PHAGOCYTOSIS :An introduction
INTRODUCTION
1) In context of immune response, Phagocytosis is a component of second line of defense .
2) Cells that perform this function are known as phagocytes.All phagocytes are either WBC or their derivatives.
3) Phagocytes ingest the microorganism and work to destroy them using variety of hydrolytic and other enzymes in a low pH environment.
4) It is highest among Neutrophiles which are WBC followed by macrophages which are derivatives of monocytes (a kind of WBC).
5) During the course of an infection, a shift occurs in the type of WBC that predominates .Neutrophils are the active phagocytes during initial phase of bacterial infections and macrophages predominate both during later phases of bacterial infection and during viral and fungal infections.
PHAGOCYTOSIS IS EXPLAINED IN OUR OTHER BLOG in detail:
PHAGOCYTOSIS :the process
1) In context of immune response, Phagocytosis is a component of second line of defense .
2) Cells that perform this function are known as phagocytes.All phagocytes are either WBC or their derivatives.
3) Phagocytes ingest the microorganism and work to destroy them using variety of hydrolytic and other enzymes in a low pH environment.
4) It is highest among Neutrophiles which are WBC followed by macrophages which are derivatives of monocytes (a kind of WBC).
5) During the course of an infection, a shift occurs in the type of WBC that predominates .Neutrophils are the active phagocytes during initial phase of bacterial infections and macrophages predominate both during later phases of bacterial infection and during viral and fungal infections.
 |
| A neutrophil cell phagocytosing an antacs bacilli |
PHAGOCYTOSIS :the process
Mechanism of phagocytosis
1) PHAGOCYTOSIS consists of 5 steps:
• Chemotaxis
• Adherence
• Ingestion
• Digestion
• Residual body formation
 |
| A simple and detailed diagram of phagocytosis |
INTERFERONS
INTERFERONS
INTERFERONS are proteins belonging to class of cytokines .although, they have the ability to protect against both bacteria, viruses, parasites and tumour. ;they are mainly known for their antiviral functions.
Different animals produce different interferons; and different cells of a given species also produce different interferons. Due to this, interferons show antiviral activity not only against viruses but also against cells of different species like that of mice or chickens.
TYPES OF INTERFERONS
Based on the type of receptor through which they signal, human interferons have been classified into three major types.
Interferon type I:
All type I IFNs bind to a cell surface receptor complex known as the IFN-α/β receptor (IFNAR) that consists of IFNAR1 and IFNAR2 Chains. In general, type I interferons are produced when the body recognizes a virus has attacked it. However, the production of type I IFN-α is prohibited by Interleukin-10. Once released, type I interferons will activate molecules which prevent the virus from producing and replicating its DNA & RNA.
Interferon type II (IFN-γ in humans):
it's production is activated by Interleukin-12 and it is released by Type 1 T helper cells. However, they block the proliferation of Type 2 T helper cells. IFN type II binds to IFNGR, which consists of IFNGR1 and IFNGR2 chains and has a different receptor than type I IFN.
Interferon type III:
Signal through a receptor complex consisting of IL10R2 (also called CRF2-4) and IFNLR1 (also called CRF2-12)
NOTE-: Expression of type I and III IFNs can be induced in virtually all cell types upon recognition of viral components, especially nucleic acids, by cytoplasmic and endosomal receptors, whereas type II interferon is induced by cytokines such as IL-12, and its expression is restricted to immune cells such as T cells and NK cells.
DOWNSTREAM SIGNALLING :
By interacting with their specific receptors, IFNs activate (STAT) complexes; different interferons can activate same or different STAT.
STAT activation initiates the (JAK-STAT) signaling pathway.
IN this pathway, IFN binds to IFN receptors outside cell and JAKs associate with IFN receptors in cytoplasm and, STAT1 &STAT2 gets activated. As a result, an IFN-stimulated gene factor 3 (ISGF3) complex forms—this contains STAT1, STAT2 and a third transcription factor called IRF9—and moves into the cell nucleus. Inside the nucleus, the ISGF3 complex binds to specific nucleotide sequences called IFN-stimulated response elements (ISREs) in the promoters of certain genes, known as IFN stimulated genes ISGs. This induces transcription of these genes and interferons are produced.
HUMAN INTERFERONS:
They are of 3 types; alpha-interferons , beta-interferons, gamma-interferons.
They are produced by lymphocytes, other leukocytes and fibroblasts in connective tissue
Interferons are small proteins with a mol.wt of 15000 to 30000.they are stable at low pH and fairly resistant to heat.
FUNCTIOND OF DIFFERENT INTERFERONS
GAMMA-INTERFERONS -it triggers phagocytosis by macrophages and neutrophils. It is produced by lymphocytes.
ALPHA & BETA-INTERFERON-they are produced by host cells infected by a virus which then diffuse to uninfected neighbouring cells and protect them from viruses and so they are said to be HOST CELL SPECIFIC and not VIRAL CELL SPECIFIC.; Because they cant act against the already infected cell...
MODE OF ACTION OF ALPHA AND BETA INTERFERONS
Virus infect the cells and start replicating there their replication induces host cell to produce mRNA for alpha and beta interferons and their further translation in to alpha and beta interferons proteins. These proteins diffuse to the neighbouring cells and degrade the viruses which infect them.
PROBLEMS WITH INTERFERONS :
They are unstable and effective for short periods. So they play prominent riles in acute and short-term viral infections like cold and influenza. But are quite ineffective against chronic and long term
IMMUNE ELEMENTS IN BLOOD
IMMUNE ELEMENTS IN BLOOD
■In response to an infection,WBC count may increase or decrease
●LEUKOCYTOSIS-an increase in WBC count occur.e.g meningitis,pneumococcal pneumonia, appendicitis, gonorrhoea and many types of bacterial infection
●LEUKOPENIA-decrease in WBC count occur .e.g.- salmonellosis ,brucellosis and some viral and rickettisial infections
●LEUKOCYTOSIS or LEUKOPENIA is dected by DIFFERENTIAL WBC COUNT which is a calculation of different types of white blood cells in a sample of 100 white blood cells.
■Leukocytes are of 2 types namely GRANULOCYTES and AGRANULOCYTES
although both contain granules but granules of granulocytes are large enough to be visible under light microscope after staining
But granules of agranulocytes cant be seen under light microscope after staining .
■BLOOD CELLS AND THEIR FUNCTIONS
●RBC-transports oxygen and carbon di oxide
●PLATELETS-blood clotting
WBC-:(GRANULOCYTES )
●Neutrophiles-phgocytosis
●Basophils-inflammation and allergy (production of histamine)
●Eosinophiles-killing of parasites by producing toxic substances
●Dendritic cells-initiation of adaptive immune response and phagocytosis
(AGRANULOCYTES )-:
●Monocytes -phagocytosis on maturation in to macrophage
●Lymphocytes (T cells,B cells, natural killer cells)
●Natural killer cells- destroy target cells by cytolysis and apoptosis
●TCells- cell mediated immunity
●B cells-humoral immunity (antibodies)
IMORTANT NOTES:
■Eosinophiles have little phagocytic activity....they mainly act on parasites like helminths....because they are very small in relation to helminths to ingest and destroy them... they attach to the outer surface of parasites and discharge peroxide ions to destroy them.
■Agranulocytes are responsible for swelling of lymph nodes during an infection.
It occurs due to maturation and proliferation of macrophages and lymphocytes.
■Natural killer cells DON'T KILL MICROBES....they ONLY KILL TARGET CELLS .
Natural killer cells bind to infected host cells. They release granules containing PERFORIN which forms channel in the plasma membrane of target cells and lead to cytolysis due to flow of extracellular fluid in it.
Second type of granules contain GRANZYMES which are protein digesting enzymes and induce the cell to undergo apoptosis.
Due to cell burst, MICROBES (may or may not be intact) are released and then killed by PHAGOCYTOSIS
■In response to an infection,WBC count may increase or decrease
●LEUKOCYTOSIS-an increase in WBC count occur.e.g meningitis,pneumococcal pneumonia, appendicitis, gonorrhoea and many types of bacterial infection
●LEUKOPENIA-decrease in WBC count occur .e.g.- salmonellosis ,brucellosis and some viral and rickettisial infections
●LEUKOCYTOSIS or LEUKOPENIA is dected by DIFFERENTIAL WBC COUNT which is a calculation of different types of white blood cells in a sample of 100 white blood cells.
■Leukocytes are of 2 types namely GRANULOCYTES and AGRANULOCYTES
although both contain granules but granules of granulocytes are large enough to be visible under light microscope after staining
But granules of agranulocytes cant be seen under light microscope after staining .
■BLOOD CELLS AND THEIR FUNCTIONS
●RBC-transports oxygen and carbon di oxide
●PLATELETS-blood clotting
WBC-:(GRANULOCYTES )
●Neutrophiles-phgocytosis
●Basophils-inflammation and allergy (production of histamine)
●Eosinophiles-killing of parasites by producing toxic substances
●Dendritic cells-initiation of adaptive immune response and phagocytosis
(AGRANULOCYTES )-:
●Monocytes -phagocytosis on maturation in to macrophage
●Lymphocytes (T cells,B cells, natural killer cells)
●Natural killer cells- destroy target cells by cytolysis and apoptosis
●TCells- cell mediated immunity
●B cells-humoral immunity (antibodies)
IMORTANT NOTES:
■Eosinophiles have little phagocytic activity....they mainly act on parasites like helminths....because they are very small in relation to helminths to ingest and destroy them... they attach to the outer surface of parasites and discharge peroxide ions to destroy them.
■Agranulocytes are responsible for swelling of lymph nodes during an infection.
It occurs due to maturation and proliferation of macrophages and lymphocytes.
■Natural killer cells DON'T KILL MICROBES....they ONLY KILL TARGET CELLS .
Natural killer cells bind to infected host cells. They release granules containing PERFORIN which forms channel in the plasma membrane of target cells and lead to cytolysis due to flow of extracellular fluid in it.
Second type of granules contain GRANZYMES which are protein digesting enzymes and induce the cell to undergo apoptosis.
Due to cell burst, MICROBES (may or may not be intact) are released and then killed by PHAGOCYTOSIS
Subscribe to:
Posts (Atom)